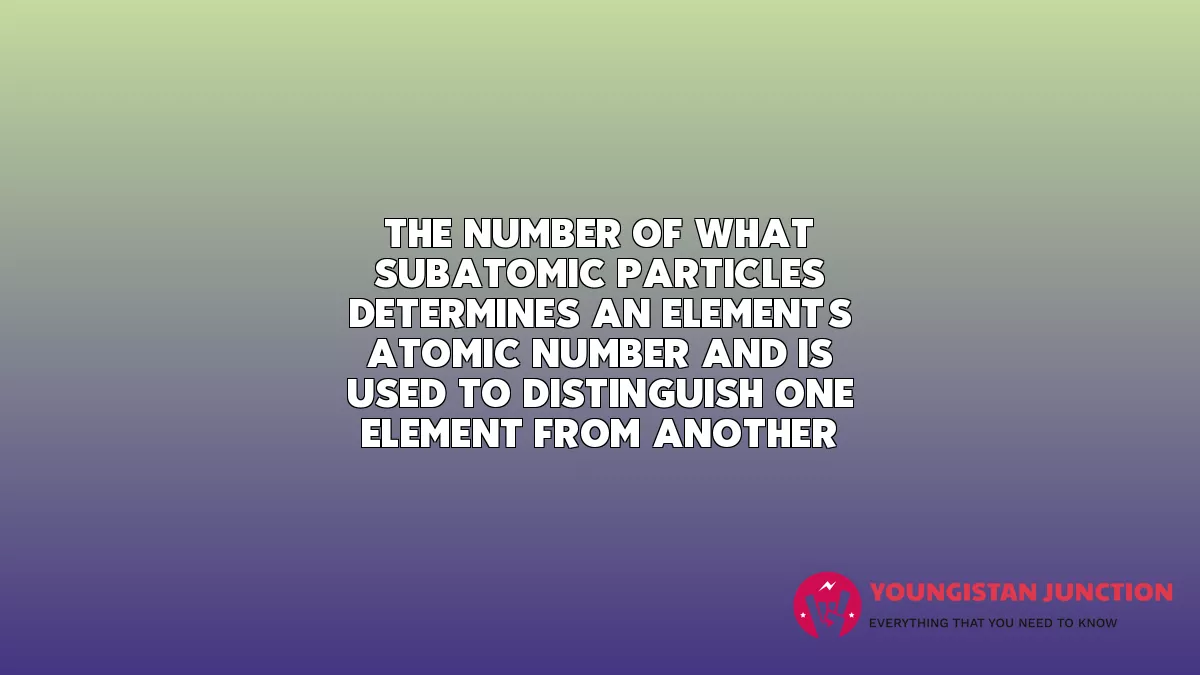
The number of what subatomic particles determines an element’s atomic number and is used to distinguish one element from another?
- Correct Answer: electrons
- neutrons
- quarks
- protons
Explanation: Atomic Number and Mass Atoms of each element contain a characteristic number of protons and electrons. The number of protons determines an element’s atomic number and is used to distinguish one element from another. The number of neutrons is variable, resulting in isotopes, which are different forms of the same atom that vary only in the number of neutrons they possess. Together, the number of protons and the number of neutrons determine an element’s mass number, as illustrated in Figure 2.3. Note that the small contribution of mass from electrons is disregarded in calculating the mass number. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Since an element’s isotopes will have slightly different mass numbers, scientists also determine the atomic mass, which is the calculated mean of the mass number for its naturally occurring isotopes. Often, the resulting number contains a fraction. For example, the atomic mass of chlorine (Cl) is 35.45 because chlorine is composed of several isotopes, some (the majority) with atomic mass 35 (17 protons and 18 neutrons) and some with atomic mass 37 (17 protons and 20 neutrons).
More Random Questions
Ans: 5
Ans: Amazon
Ans: Protection from +20°C to -60°C
Ans: Shaurya Saini
Ans: Valsanabha
Ans: Assam
Ans: Uro Shoola
Ans: Diana Pundole
Ans: chemical
Ans: Odisha
Ans: crustal cells
Ans: West Indies
Ans: 47%
Ans: Myocardial infarction
Ans: Sweden